Wallaby Medical Esperance™ 6F Aspiration Catheter Approved by NMPA
Date:2022-04-02
LAGUNA HILLS, CA and SHANGHAI - April 02, 2022, The Esperance™ 6F Aspiration Catheter independently developed by Wallaby Medical was approved by the China National Medical Products Administration (NMPA) to be marketed in China.
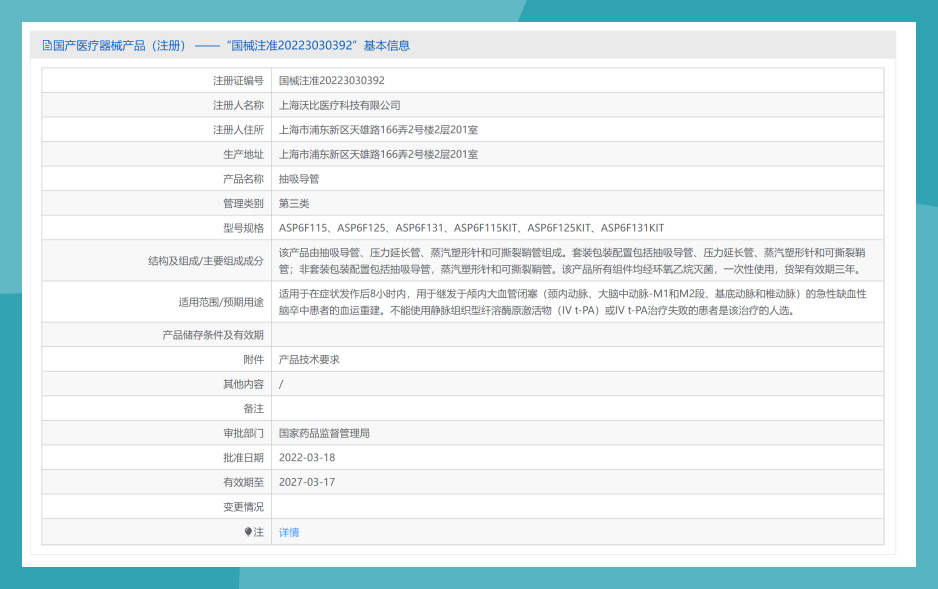
Figure 1: Esperance™ 6F NMPA Certificate
Figure 2: Esperance™ 6F Aspiration Catheter
In August 2021, the National Health Commission issued the Guidelines for the Prevention and Treatment of Stroke in China (2021 Edition), in which it is emphasized that stroke has become the number one cause of death in China, with high morbidity, high disability rate, high mortality and high recurrence rate. The guidelines indicate it is urgent to comprehensively promote the prevention and treatment of stroke. In China, Acute ischemic stroke (AIS) accounts for about 70% of all cases of stroke. From intravenous thrombolysis, intravenous thrombolysis combined with mechanical thrombectomy, to mechanical thrombectomy alone, mechanical thrombectomy devices have increasingly gained popularity. In recent years, a number of international clinical research reports have shown that aspiration thrombectomy enjoys higher complete vascular recanalization rate and shorter vascular reperfusion time under certain circumstances compared with stent retrieval thrombectomy. At the same time, the safety data, such as adverse event rates of aspiration thrombectomy, are also similar to those of stent retriever thrombectomy. Also, the combination of aspiration thrombectomy and stent retrievers is increasingly used for complex lesions.
"Esperance" means "hope" in French. Sticking to the original design intention of "Focusing on first-pass recanalization", Wallaby Medical intends the Esperance™ aspiration catheter to bring hope to more acute ischemic stroke patients around the world.
Prof. Jiao Liqun, Director of Interventional Radiology Department and Deputy Director of the Neurosurgery Department at Xuanwu Hospital of Capital Medical University, said, "It is safe and effective to use Wallaby Medical Esperance™ 6F aspiration catheter as a first-line aspiration method based on our domestic multi-center registration clinical trial results. The one-time recanalization rate of Esperance™ 6F aspiration catheter is as high as 65%, which is 57% higher than in the aspiration group of COMPASS study [1]. Thanks to its rapid and efficient first-pass recanalization, the 90-day mRS (0-2) of the enrolled patients reached 62.3%. This aspiration catheter achieves high reperfusion rates with fewer salvage stent retriever treatments.
Esperance™ is designed to achieve fast access to cerebral vessels. This is possible due to its advanced design with coil and braid combined with tailored zones of flexibility and highly lubricious hydrophilic coating along the catheter's shaft. Esperance's large 0,071" PTFE lined lumen results in high aspiration force resulting in improved first-pass recanalization rate.
Since the establishment of the company, Wallaby Medical has actively responded to the Healthy China 2030 Plan, empowering medical institutions in the field of neuro intervention with a strong portfolio of medical devices and benefiting domestic stroke patients. So far, in addition to Esperance™ aspiration catheter, Avenir® coil system has also been approved by NMPA for marketing in China.
Source:
[1] Turk AS,Siddiqui A,Fifi JT,et al.Aspiration thrombectomy versus stent retriever thrombectomy as first-line approach for large vessel occlusion (COMPASS):a multicentre,randomised,open label, blinded outcome,non-inferiority trial. Lancet. 2019; 393: 998-1008.
 中文版
中文版
 Official Wechat
Official Wechat